Reinigungsvalidierung – einfach gemacht.
CLEEN wurde von Experten entwickelt, die die Herausforderungen von Kontaminationskontrolle, Compliance und pharmazeutischer Fertigung selbst erlebt haben und ihre Praxiserfahrung in jede Funktion einbringen.

Pharma 4.0
Warum die betriebliche Digitalisierung für Pharma- und Biopharma-Hersteller oberste Priorität haben muss.
Dieses kostenlose eBook beschreibt die grundlegenden Veränderungen, die mit der Einführung von Pharma 4.0 einhergehen – und wie diese Unternehmen dabei unterstützen können, Effizienz und Agilität zu steigern, die Produktqualität zu sichern und gleichzeitig die Unternehmensprofitabilität zu erhöhen.
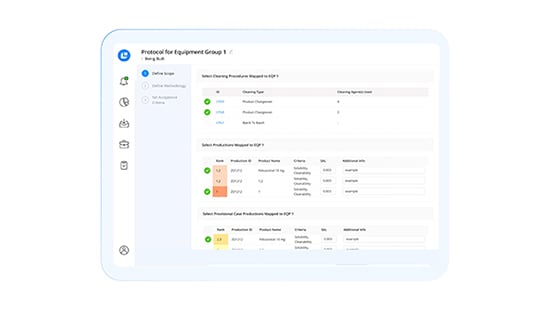
Modernisieren Sie die Reinigungsvalidierung mit intelligenter Automatisierung
Fügen Sie hier eine Beschreibung und den SEO-Untertitel hinzu
Gewährleistung konformer Validierungen
Profitieren Sie von plattforminternen Anweisungen zur Erstellung von Validierungen von Reinigungsabläufen auf der Grundlage ständig aktualisierter Vorschriften. Berechnen Sie automatisch Verschleppungsgrenzen sowie Worst-Case-Produktklassifizierungen und nutzen Sie automatische Compliance-Sperren für CHT/DHT.
Beschleunigte Re-Validierung
Nutzen Sie historische Daten, um Verschleppungsgrenzen zu berechnen und Protokolle in wenigen Minuten zu erstellen, indem Sie Daten aus mehreren Quellen integrieren, um die Einführung neuer Produkte zu beschleunigen. Erhalten Sie dynamische Benachrichtigungen für notwendige erneute Validierungen mit automatisch generierten Dokumenten zur Überprüfung.
Vereinfachung der Bereitschaft für Audits
Erstellen Sie Validierungsberichte automatisch und speichern Sie alle erforderlichen Informationen im integrierten Audit-Portal für den unmittelbaren Zugriff, der eine kontinuierliche Bereitschaft für Audits bei minimalem Verwaltungsaufwand ermöglicht.
Hauptmerkmale
Genaue Berechnung von Rückstandsgrenzwerten auf Basis von HBEL sicherstellen
Gesundheitsbasierte Expositionsgrenzwerte (Health-Based Exposure Limits, HBEL) sind wissenschaftlich abgeleitete Grenzwerte, die den maximal zulässigen Übertrag von pharmazeutischen Rückständen auf gemeinsam genutzten Produktionsanlagen festlegen.
- Berechnen Sie anhand von HBEL-Daten automatisch sichere Rückstandsgrenzwerte, um die Einhaltung des Worst-Case-MACO (maximal zulässiger Übertrag) sicherzustellen.
- CLEEN unterstützt die risikobasierte Reinigungsvalidierung in der Pharmaindustrie, die den Anforderungen der FDA und des ICH entspricht.

Risikobewertung der Einführung neuer Arzneimittel
Bewerten Sie die Auswirkungen der Integration neuer Arzneimittel in bestehende Produktionslinien. Bestimmen Sie sofort Übertragungsgrenzwerte, Risikostufen und Worst-Case-Szenarien. Die Risikomatrix von CLEEN unterstützt die Reinigungsvalidierung, gewährleistet die Einhaltung gesetzlicher Vorschriften und steigert die betriebliche Effizienz.
- Sofortige Auswirkungsanalyse zur Bewertung von Kreuzkontaminationsrisiken in gemeinsam genutzten Einrichtungen.
- Validierung analytischer Methoden, insbesondere in Bezug auf die Nachweisgrenze (LOD).
- Vorhersage künftiger Risiken und Aktualisierung der Parameter für die Reinigungsvalidierung.

Automatische Generierung standardisierter, konformer Validierungsprotokolle für die Reinigung
- Automatische Erstellung von Protokollen unter Verwendung integrierter Risikoanalysen, HBEL-Daten und historischer Rückstandsprofile.
- Unterstützt die Berechnung von Übertragungsgrenzwerten für Wirkstoffe, Reinigungsmittel, Mikroorganismen und Nitrosamine.
- Ermöglicht die Erstellung von Protokollen mit nur einem Klick durch die Aggregation von Risikoanalysen, Analysemethoden und Toxizitätsdaten.
- Bietet Echtzeitwarnungen, wenn neue Validierungen erforderlich sind, und gewährleistet so die Kontinuität der Compliance.

Integrieren Sie Rückstandsdaten aus LIMS, um Berichte zu erstellen
- Importiert Rückstandsergebnisse aus LIMS direkt in die Validierungsplattform.
- Erstellt umfassende Berichte unter Verwendung vorkonfigurierter Vorlagen.
- Unterstützt die automatische Erstellung von Protokollen, die Berechnung der Rückstandsgrenzen und die Verfolgung von Validierungen in Echtzeit.

Verfolgen Sie den Validierungsstatus aller Geräte in Echtzeit
- Verfolgt den Validierungsstatus aller Geräte in Echtzeit.
- Visuelle Unterscheidung zwischen abgeschlossenen, laufenden und ausstehenden Validierungen.
- Lässt sich in Planungstools integrieren, um mehrere Studien gleichzeitig zu verwalten.
- Unterstützt anpassbare Berichte für die FDA.

Nutzung des Audit-Portals bei Inspektionen
- Sofortiger Zugriff auf Validierungsberichte, SOPs, Protokolle und Begleitdokumente.
- Digital signierte, Part 11-konforme Protokolle für Rückverfolgbarkeit und Regelkonformität.
- Führen Sie einen vollständigen Prüfpfad über Änderungen, Validierungen und Bedieneraktionen.
- Ermöglicht externen Prüfern den sicheren Zugriff auf relevante Daten, ohne nicht verwandte Systeme offenzulegen.
- Behördliche Inspektionen: Rufen Sie produktspezifische Berichte auf, um einen 360°-Überblick über die Reinigungsleistung zu erhalten.

Häufig gestellte Fragen
Konforme und skalierbare Cloud-Infrastruktur
CLEEN automatisiert MACO-Berechnungen, Bewertungen von Rückstandsgrenzwerten und standardisierte Berichte, um sie an die Reinigungsvalidierungsstandards der FDA, EMA und ICH anzupassen. Die auditfähige Dokumentation stellt sicher, dass Sie jederzeit auf Inspektionen vorbereitet sind.
Ja. CLEEN ist so konzipiert, dass es sich nahtlos in Ihre aktuellen Qualitätsmanagementsysteme, Laborinformationsmanagementsysteme und ERPs integrieren lässt, wodurch Datensilos beseitigt und ein vernetztes Validierungs-Ökosystem gewährleistet werden.
CLEEN bietet durchgehende Automatisierung, Echtzeit-Tracking und konfigurierbare Workflows, die speziell für die pharmazeutische Industrie entwickelt wurden. Es wurde mit Fokus auf Audit-Bereitschaft und Regelkonformität entwickelt – hier gibt es keine generischen Lösungen.
Absolut. CLEEN erfüllt vollständig 21 CFR Part 11 und bietet sichere Audit-Trails, elektronische Signaturen, rollenbasierte Zugriffskontrolle sowie Datenverschlüsselung, um alle globalen Anforderungen an die Datenintegrität zu erfüllen.
CLEEN ermöglicht es Ihnen, Risiken auf der Grundlage von Produkttoxizität, Löslichkeit, Chargengröße und mehr zu bewerten und Kreuzkontaminationen zu verhindern. Es berechnet automatisch Worst-Case-Szenarien und unterstützt ICH- und APIC-Frameworks für die Reinigungsvalidierung.
Kunden können in der Regel den manuellen Validierungsaufwand um bis zu 60 % reduzieren, die Audit-Vorbereitung um 80 % beschleunigen und die Compliance-Risiken deutlich verringern. CLEEN spart zudem Kosten, indem Dokumentationsprozesse optimiert und Nacharbeiten reduziert werden.
Die Einführung hängt von der Größe Ihrer Einrichtung und den Systemintegrationen ab. Die meisten Kunden sind innerhalb von 4 bis 8 Wochen vollständig betriebsbereit, unterstützt durch unsere speziellen Onboarding- und Validierungsteams.
Ja. CLEEN ist eine sichere, cloud-basierte Plattform, die von überall aus zugänglich ist, Echtzeit-Zusammenarbeit über globale Teams ermöglicht und gleichzeitig die Einhaltung gesetzlicher Vorschriften sicherstellt.
Ja. CLEEN bietet praxisnahes Onboarding, Live-Schulungen, detaillierte Dokumentation sowie 24/7 Kundensupport, um sicherzustellen, dass Ihr Team schnell und kompetent einsatzbereit ist.
Absolut. Sie können eine personalisierte Demo anfordern, die auf die Bedürfnisse Ihrer Einrichtung zugeschnitten ist. Wir zeigen Ihnen, wie sich CLEEN in Ihre bestehenden Arbeitsabläufe und Ihre regulatorische Strategie einfügt.


